
Alain Garcia De Las Bayonas, PhD
How did single cells turn into animals ?
Hi, I am Alain!
I am a postdoctoral researcher in the lab of Professor Nicole King at UC Berkeley, CA. I study various aspects of the biology of fascinating organisms: the choanoflagellates. Choanoflagellates are the closest living relatives of animals and therefore can help us understand how animals first evolved!
I received my PhD from Aix-Marseille University, France, working in the lab of Professor Thomas Lecuit. My thesis work focused on elucidating signaling pathways controlling cell shape changes in the developing Drosophila embryo.
In my free time I enjoy drawing, hiking and looking for arthropods in the bay area.
email: alaingdlb@berkeley.edu
CURRENT RESEARCH
Choanoflagellates are the closest living single-celled and colony forming relatives of animals. By comparing the genome and cell biology of these organisms with the ones of animals, we can infer the origins and evolution of Metazoan. My postdoctoral work organizes around two research axes:
(1) Diversity and Function of GPCR families in choanoflagellates
G protein-coupled receptors (GPCRs) are fundamental to animal biology where they have been extensively studied. In contrast, little is known about the diversity and function of GPCRs which predate animal origins. In this project, I will take advantage of the genome and/or transcriptome of 24 choanoflagellate species as well as our recent ability to perform CRISPR–Cas9 gene editing in the model choanoflagellate Salpingoeca rosetta to assess the diversity and function GPCRs in choanoflagellates. This work promises to uncover the ancestral function of conserved GPCR families in Metazoa.
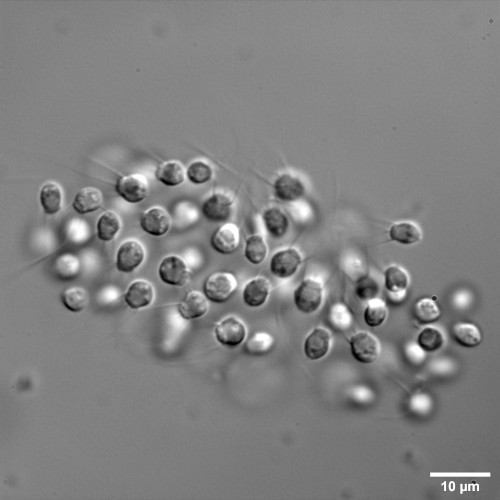
CRISPR-Cas9 targeted knock-out of a GPCR triggers cell aggregation in Salpingoeca rosetta.
(2) Colony morphogenesis in the choanoflagellate B. monosierra
Choanoflagellates can form multicellular structures called rosettes when exposed to specific environmental cues. Rosettes result from clonal division and resemble the blastula stage of animal development. Understanding the principles underlying rosette formation in choanoflagellates could help us understanding the evolution of animal multicellularity. Barroeca monosierra, a colonial choanoflagellate recently isolated from Mono Lake (CA), forms among the largest rosettes described in choanoflagellates. Preliminary observations suggest that B. monosierra colonies are dynamic structures whose morphogenesis has not been investigated at the cellular level. Additionally, B. monosierra colonies contain a microbiome, providing a unique opportunity to assess the effect of microbial communities on rosette development.
Two happily swimming B. monosierra colonies confined in a microgrid array.
CHOANO - MICROSCOPY
How can we learn more about the cell biology of choanoflagellates ? By looking at them! You will find in
this section some nice videos and pictures I have been taking of choanoflagellates. I will (try to) keep this
section updated.
Choanoflagellates possess a flagellum surrounded by a collar of actin-rich microvilli. The collar is a very fragile structure, often compromised when using a fixative. I developed a protocol to reveal the ultrastructure of the choanoflagellate collar in living cells!
Baroecca. monosierra rosette at high magnification (100x). The flagellum of the cells point outward from the colony. A dense extracellular matrix fills the center of the colony.
PAST RESEARCH
Spatial control over Rho1 signaling during epithelial morphogenesis
RhoGTPases are tightly regulated in space and time to specify the desired pattern of actomyosin contractility that supports tissue morphogenesis. How is the activity of RhoGTPases controlled in developing epithelia remains unclear. During my PhD, I have examined this general problem in the context of cell intercalation that drives extension of the Drosophila ectoderm. By combining a genetic screen with the use of a Rho1-GTP biosensor and live imaging approaches, I identified two RhoGEFs which independently activate Rho1 at the medio-apical and junctional compartments in intercalating cells. This process requires upstream control by distinct heterotrimeric G proteins.
Knockdown of Dp114-RhoGEF leads to a specific decrease in junctional Rho1 signaling (here Myo-II:mCh) in the Drosophila embryo.
© Alain Garcia De Las Bayonas, PhD